PH FULL FORM
Full Form of PH : Full Form of PH of Poyential of Hydrogen.
What is the full form of PH?
No:1. The full form of PH is Potential of Hydrogen.
No:2. It is known as the negative logarithm of H+ ions concentration.
No:3. Therefore the meaning of the name pH (Potential of Hydrogen) is explained as the strength of hydrogen. Potential of Hydrogen) pH describes the concentration of the H+ ions (hydrogen ions) in a solution.
No:4. pH is the indicator of acidity or basicity of the solution.
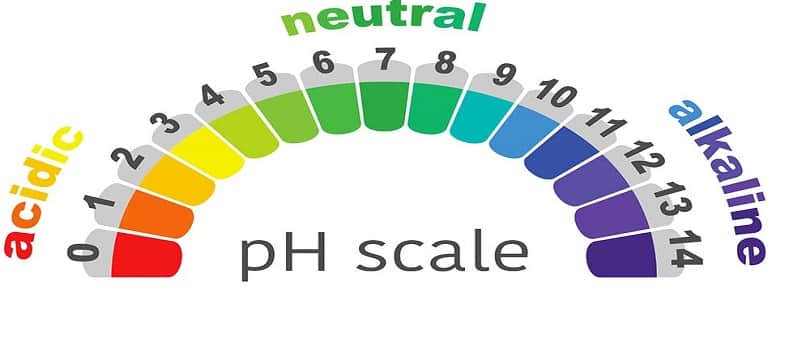
No:5. The pH value varies from 0 to 14 on a pH scale.
No:6. The concentration of hydronium ions is conveniently expressed on a logarithmic scale.
No:7. Logarithm scale is known as the pH scale.
No:8. pH of acids and bases is defined as the negative logarithm (with base 10) of activity of H+ ions (hydrogen ions).
No.-1. Download 15000 One Liner Question Answers PDF
No.-2. Free Download 25000 MCQ Question Answers PDF
No.-3. Complete Static GK with Video MCQ Quiz PDF Download
No.-4. Download 1800+ Exam Wise Mock Test PDF
No.-5. Exam Wise Complete PDF Notes According Syllabus
No.-6. Last One Year Current Affairs PDF Download
No.-7. Join Our Whatsapp Group
No.-8. Join Our Telegram Groupv
pH value of Acid And Base
No:1. The PH of the solution has a range from 0 to 14.
No:2. All the solutions with a pH value varying from 0 – 7 on the pH scale are called acidic solutions.
No:3. All the solutions with a pH value varying from 7 – 14 on the pH scale are known as basic solutions.
No:4. All the solutions with a potential of hydrogen value equal to 7 are known as neutral solutions.
No:5. The solutions with a pH-value of 0 are considered to be highly acidic.
No:6. The acidity decreases as the pH value increase from 0 to 7 while the solutions with a pH value equal to 14 are known to be highly basic or strongly alkaline solutions.
No:7. The acid and base intensity depends on the number of H+ and OH– ions produced.
No:8. Acids furnishing more H+ ions are known to be strong acids and vice versa.
Importance of pH
No:1. A living organism can withstand only a limited range of pH changes, and any more pH adjustments will make life difficult.
No:2. For example: In case of acid rain, the pH value of water is less than 7 which increases the pH of river water as it flows into a river which effects the survival of marine life.
No:3. The human stomach contains HCL (hydrochloric acid) which helps the digestion of the food.
No:4. The stomach releases so much HCl we feel a lot of pain and discomfort during indigestion. It would be minimise by using antacids.
No:5. The bacteria present in our mouth lower the pH of our mouth by generating acids by food particle degradation.
No:6. Hence by maintaining the pH, we are told to clean our mouths with toothpaste that are basic to prevent their decay.
No:7. In the case of bee-sting, we feel a lot of pain when the bee injects the methanoic acid through its sting. Therefore, we are usually recommended to apply baking soda or other mild bases to the surface because it helps to maintain the surface pH .
MUST READ : ECE FULL FORM
No.-1. Download 15000 One Liner Question Answers PDF
No.-2. Free Download 25000 MCQ Question Answers PDF
No.-3. Complete Static GK with Video MCQ Quiz PDF Download
No.-4. Download 1800+ Exam Wise Mock Test PDF
No.-5. Exam Wise Complete PDF Notes According Syllabus
No.-6. Last One Year Current Affairs PDF Download
No.-7. Join Our Whatsapp Group
No.-8. Join Our Telegram Group